Current Projects
Leveraging mice with natural microbial exposures to redefine osteoporosis pathogenesis
Premise: Specific pathogen-free (SPF) mice lack exposure to pathogenic and commensal microbes and form the foundation of biomedical research. If you do work with laboratory animals, the chances are extremely high that they are SPF. Recent work from immunology (e.g. Beura et al. 2016 in Nature, Hamilton et al. 2020 in J Clin Immunol) and microbiology (e.g. Rosshart et al. 2019 in Science) have called into question the utility of the SPF model, as microbial exposures robustly impact the immune system and therefore, broader host physiology. Unlike immune naïve SPF mice, mice with natural microbial exposures (NME; e.g. wild or pet store mice) are immune mature, demonstrating chronic immune activation and paralleling basal inflammatory responses seen in humans.
Bone-Immune Link: Bone and the immune system are intricately linked, particularly in the bone marrow microenvironment where immune cells are housed and generated. Microbial exposures activate immune cells (e.g. T cells), which then produce inflammatory cytokines (e.g. TNFa, IFNy, interleukins, etc.) to coordinate the immune response. Both immune cells and their inflammatory cytokines are major regulators of bone and bone cells, including osteoblasts and mesenchymal stem cells.
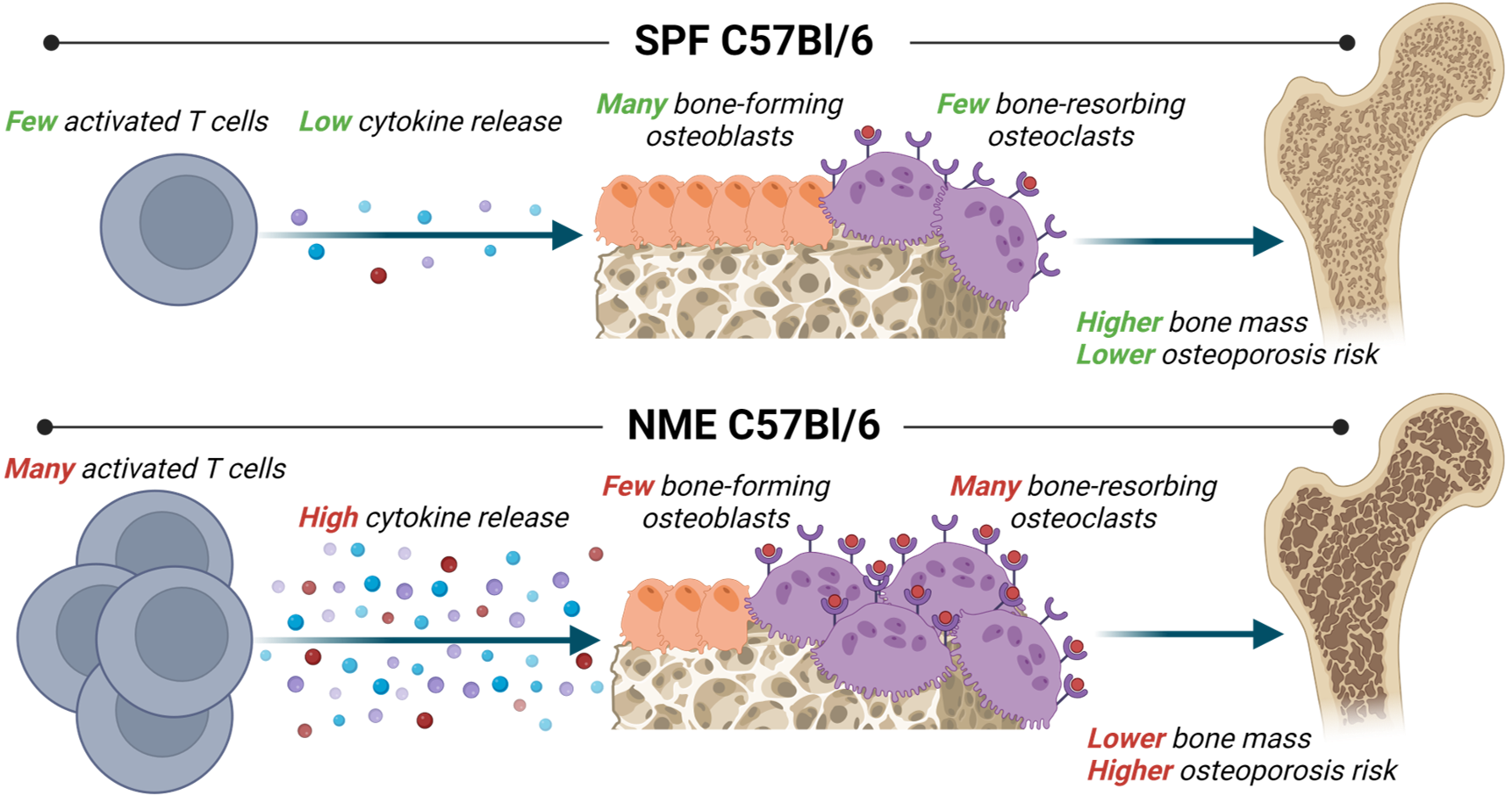
NME mice have significantly elevated levels of over 20 inflammatory cytokines known to critically mediate bone homeostasis, indicating the cell signaling environment in NME mice is primed to fundamentally alter bone homeostasis relative to an immune naïve animal – see the image for a visual summary. For a detailed explanation of the NME mouse and historical context of the field of osteoimmunology, see Little-Letsinger & Hamilton 2023 in Frontiers in Endocrinology.
Current Status: This work is on-going! Our efforts are primarily related to developing a model that recapitulates human bone homeostasis and bone marrow composition. Our overarching goal is to leverage this novel translational model to redefine osteoporosis pathogenesis under the premise that osteoporosis is an immune-mediated disease. While this is our primary goal, our interests are broad and we are always looking for new collaborations!
Future Directions: Future work with the NME mouse includes, but is not limited to the following.
- Explore the utility of the NME model for bone marrow metastases
Experimental evolution of the skeleton and resulting trade-offs with the osteoimmune-reproductive axis
Premise: Modern humans are the only animals that experience spontaneous fracture. The skeleton of modern humans is gracile, demonstrating reduced cortical and trabecular bone mass and strength, relative to earlier human ancestors. Our reduced bone mass and elevated fracture risk likely derives from impaired bone mass acquisition during adolescence, a critical period of skeletal development during which 90% of bone mass accrues. The behavioral, environmental, and physiological factors responsible for the divergence in bone mass acquisition between modern humans and our ancestors remain unknown.
Connecting to the Neolithic: Skeletal gracilization temporally associates with the Neolithic period, marked by the transition from foraging to farming that ultimately led to massive increases in infectious disease burden to adoption of permanent dwellings, domestication of livestock, and increasing population density. Current hypotheses regarding skeletal gracilization posit physical inactivity as the primary driver (e.g. see work from Ruff, Chirchir, Ryan and others). However, skeletal gracilization has stabilized from the Neolithic through to the 20th century. Given the effects of industrialization and record high rates of physical inactivity among the current population, further gracilization would be expected if physical inactivity were the primary driver.
The Neolithic is also considered to be a “turning point” in the evolution of the immune system (Dominguez-Andres et al. 2021 eLife). Sustained increases in population density, poor sanitary conditions, and greater contact between humans and animals enabled robust increases in infectious and zoonotic diseases. Among modern day farming communities with high rates of physical activity (e.g. Tsimane, Shuar), those communities with high infectious disease burden demonstrate bone loss and fracture rates that exceed that of US citizens, despite the absence of risk factors common in industrialized society. When considering the importance of the immune system for regulation of bone homeostasis, the rise in infectious disease burden during the Neolithic is a promising avenue to understand skeletal gracilization and the emergence of spontaneous fracture. For a detailed explanation of the role of infectious disease in controlling bone homeostasis in the evolution of the human skeleton, see Little-Letsinger & Hamilton 2023 in Frontiers in Endocrinology.
Current Status: This work is on-going! Our efforts are primarily related to artificial selection relating to immune defense (using the NME mouse described above) and reproductive fitness. We are focused on the trade-offs within and across systems, considering the skeletal, immune, and reproductive systems, and within and across generations. If this work excites you, please do not hesitate to reach out as we are always looking for new collaborations!
Future Directions: Future work with the NME mouse includes, but is not limited to the following.
- Explore the future evolution of bone in the context of adapting to a multi-planetary existence, specifically in resistance to radiation exposure and microgravity
Overview of Future Directions:
Future projects are always under consideration, and include, but are not limited to:
- Bone health in space flight – considering microgravity and/or radiation
- How aging of the immune system impacts bone health
- Sex hormone-regulation of bone health in adolescence and post-menopause
- Dietary regulation of bone health – including diet-induced obesity and caloric restriction
- Effect of habitual movement patterns on bone strength and structure
- Others….
We are always eager to discuss new or existing ideas and directions with interested parties!
